VIVO Pathophysiology
Thyroid Hormones: Pregnancy and Fetal Development
Thyroid hormones are critical for development of the fetal and neonatal brain, as well as for many other aspects of pregnancy and fetal growth. Hypothyroidism in either the mother or fetus frequently results in fetal disease; in humans, this includes a high incidence of mental retardation.
Maternal Thyroid Function During Pregnancy
Normal pregnancy entails substantial changes in thyroid function in all animals. These phenomena have been studied most extensively in humans, but probably are similar in all mammals. Major alterations in the thyroid system during pregnancy include:
- Increased blood concentrations of T4-binding globulin: TBG is one of several proteins that transport thyroid hormones in blood, and has the highest affinity for T4 (thyroxine) of the group. Estrogens stimulate expression of TBG in liver, and the normal rise in estrogen during pregnancy induces roughly a doubling in serum TBG concentratrations.
- Increased levels of TBG lead to lowered free T4 concentrations, which results in elevated TSH secretion by the pituitary and, consequently, enhanced production and secretion of thyroid hormones. The net effect of elevated TBG synthesis is to force a new equilibrium between free and bound thyroid hormones and thus a significant increase in total T4 and T3 levels. The increased demand for thyroid hormones is reached by about 20 weeks of gestation and persists until term.
- Increased demand for iodine: This results from a significant pregnancy-associated increase in iodide clearance by the kidney (due to increased glomerular filtration rate), and siphoning of maternal iodide by the fetus. The World Health Organization recommends increasing iodine intake from the standard 100 to 150 ug/day to at least 200 ug/day during pregnancy.
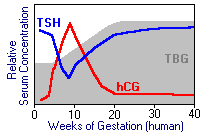 Thyroid stimulation by chorionic gonadotropin: The placentae of humans and other primates secrete huge amounts of a hormone called chorionic gonadotropin (in the case of humans, human chorionic gonadotropin or hCG) which is very closely related to luteinizing hormone. TSH and hCG are similar enough that hCG can bind and transduce signalling from the TSH receptor on thyroid epithelial cells. Toward the end of the first trimester of pregnancy in humans, when hCG levels are highest, a significant fraction of the thyroid-stimulating activity is from hCG. During this time, blood levels of TSH often are suppressed, as depicted in the figure to the right. The thyroid-stimulating activity of hCG actually causes some women to develop transient hyperthyroidism.
Thyroid stimulation by chorionic gonadotropin: The placentae of humans and other primates secrete huge amounts of a hormone called chorionic gonadotropin (in the case of humans, human chorionic gonadotropin or hCG) which is very closely related to luteinizing hormone. TSH and hCG are similar enough that hCG can bind and transduce signalling from the TSH receptor on thyroid epithelial cells. Toward the end of the first trimester of pregnancy in humans, when hCG levels are highest, a significant fraction of the thyroid-stimulating activity is from hCG. During this time, blood levels of TSH often are suppressed, as depicted in the figure to the right. The thyroid-stimulating activity of hCG actually causes some women to develop transient hyperthyroidism.
The net effect of pregnancy is an increased demand on the thyroid gland. In the normal individuals, this does not appear to represent much of a load to the thyroid gland, but in females with subclinical hypothyroidism, the extra demands of pregnancy can precipitate clinicial disease.
Thyroid Hormones and Fetal Brain Development
In 1888 the Clinical Society of London issued a report underlining the importance of normal thyroid function on development of the brain. Since that time, numerous studies with rats, sheep and humans have reinforced this concept, usually by study of the effects of fetal and/or maternal thyroid deficiency. Thyroid hormones appear to have their most profound effects on the terminal stages of brain differentiation, including synaptogenesis, growth of dendrites and axons, myelination and neuronal migration.
Thyroid hormones act by binding to nuclear receptors and modulating transcription of responsive genes. Thyroid hormone receptors are widely distributed in the fetal brain, and present prior to the time the fetus is able to synthesize thyroid hormones. It has proven surprisingly difficult to identify the molecular targets for thyroid hormone action in the developing brain, but some progress has been made. For example, the promoter of the myelin basic protein gene is directly responsive to thyroid hormones and contains the expected hormone response element. This fits with the observation that induced hypothyroidism in rats leads to diminished synthesis of mRNAs for several myelin-associated proteins.
It seems clear that there is a great deal more to learn about the molecular mechanisms by which thyroid hormones support normal development of the brain.
Thyroid Deficiency in the Fetus and Neonate
The fetus has two potential sources of thyroid hormones - it's own thyroid and the thyroid of it's mother. Human fetuses acquire the ability to synthesize thyroid hormones at roughly 12 weeks of gestation, and fetuses from other species at developmentally similar times. Current evidence from several species indicates that there is substantial transfer of maternal thyroid hormones across the placenta. Additionally, the placenta contains deiodinases that can convert T4 to T3.
There are three types or combinations of thyroid deficiency states known to impact fetal development:
Isolated maternal hypothyroidism: Overt maternal hypothyroidism typically is not a significant cause of fetal disease because it usually is associated with infertility. When pregnancy does occur, there is increased risk of intrauterine fetal death and gestational hypertension. Subclincial hypothyroidism is increasingly being recognized as a cause of developmental disease - this is a rather scary situation. Several investigators have found that mild maternal hypothyroidism, diagnosed only retrospectively from banked serum, may adversely affect the fetus, leading in children to such effects as slightly lower performance on IQ tests and difficulties with schoolwork. The most common cause of subclinical hypothyroidism is autoimmune disease, and it is known that anti-thyroid antibodies cross the human placenta. Thus, the cause of this disorder may be a passive immune attack on the fetal thyroid gland.
Isolated fetal hypothyroidism: This condition is also known as sporadic congenital hypothyroidism. It is due to failure of the fetal thyroid gland to produce adequate amounts of thyroid hormone. Most children with this disorder are normal at birth, because maternal thyroid hormones are transported across the placenta during gestation. What is absolutely critical is to identify and treat this condition very shortly after birth. If treatment is not instituted quickly, the child will become permanently mentally and growth retarded - a disorder called cretinism. This problem has largely disappeared in the US and many other countries due to large scale screening programs to detect hypothyroid infants.
Iodine deficiency - Combined maternal and fetal hypothyroidism: Iodine deficiency is, by a large margin, the most common preventable cause of mental retardation in the world. Without adequate maternal iodine intake, both the fetus and mother are hypothyroid, and if supplemental iodine is not provided, the child may well develop cretinism, with mental retardation, deaf-mutism and spasticity.
The World Health Organization estimated in 1990 that 20 million people had some degree of brain damage due to iodine deficiency experienced in fetal life. Endemic iodine deficiency remains a substantial public health problem in many parts of the world, including many areas in Europe, Asia, Africa and South America. In areas of severe deficiency, a large fraction of the adult population may show goiters. In such settings, overt cretinism may occur in 5 to 10 percent of offspring, and perhaps five times that many children will have mild mental retardation. This is a serious, tragic and, most importantly, a preventable problem.
The effects of mild maternal hypothyroidism on cognitive function of children has been evaluated in several studies, including some in which mothers will low levels of T4 or high levels of TSH were treated prophylactically with thyroid supplementation. The results of these studies are somewhat divergent, and the benefit of routinely testing pregnant women and treating those with suspected thyroid deficiency remains unsettled.
The fetus of an iodine-deficient mother can be successfully treated if iodine supplementation is given during the first or second trimester. Treatment during the third trimester or after birth will not prevent the mental defects.
Iodine deficiency can also be a sigificant problem in animal populations. The most common manifestation in sheep, cattle, pigs and horses is a high incidence of stillbirths and birth of small, weak offspring.
Hyperthyroidism in Pregnancy
Gestational hyperthyroidism is associated with increased risk of several adverse outcomes, including preeclampisa, premature labor, fetal or perinatal death and low birth weight. In humans, hyperthyroidism usually is the result of Grave's disease, which involves development of autoantibodies against the TSH receptor that stimulate the thyroid gland.
References and Reviews
- Brent GA. The debate over thyroid-function screening in pregnancy. New Eng J Med 2012; 366:562-563.
- Glinoer D: The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev 18:404, 1997.
- Haddow JE, Palomaki GE, Allan WC, et al: Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. New Eng J Med 341:549-555, 1999.
- Lazarus JH, Bestwick JP, Channon S, et al: Antenatal thyroid screening and childhood cognitive function. New Eng J Med 2012; 366:493-498.
- Oppenheimer JH, Schwartz HL: Molecular basis of thyroid hormone-dependent brain development. Endocr Rev 18:462-475, 1997.
- Xue-Yi C, Xin-Min J, Zhi-Hong D, et al: Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. New Eng J Med 221:1739-1744, 1994.
Send comments to Richard.Bowen@colostate.edu
