VIVO Pathophysiology
Thyroid Hormone Receptors
Receptors for thyroid hormones are members of a large family of nuclear receptors that include those of the steroid hormones. They function as hormone-activated transcription factors and thereby act by modulating gene expression. In contrast to steroid hormone receptors, thyroid hormone receptors bind DNA in the absence of hormone, usually leading to transcriptional repression. Hormone binding is associated with a conformational change in the receptor that causes it to function as a transcriptional activator.
Receptor Structure
Mammalian thyroid hormone receptors are encoded by two genes, designated alpha and beta. Further, the primary transcript for each gene can be alternatively spliced, generating different alpha and beta receptor isoforms. Currently, four different thyroid hormone receptors are recognized: alpha-1, alpha-2, beta-1 and beta-2.
Like other members of the nuclear receptor superfamily, thyroid hormone receptors encapsulate three functional domains:
- A transactivation domain at the amino terminus that interacts with other transcription factors to form complexes that repress or activate transcription. There is considerable divergence in sequence of the transactivation domains of alpha and beta isoforms and between the two beta isoforms of the receptor.
- A DNA-binding domain that binds to sequences of promoter DNA known as hormone response elements.
- A ligand-binding and dimerization domain at the carboxy-terminus.
As depicted in the figure below, the DNA-binding domains of the different receptor isoforms are very similar, but there is considerable divergence among transactivation and ligand-binding domains. Most notably, the alpha-2 isoform has a unique carboxy-terminus and does not bind triiodothyronine (T3).

The different forms of thyroid receptors have patterns of expression that vary by tissue and by developmental stage. For example, almost all tissues express the alpha-1, alpha-2 and beta-1 isoforms, but beta-2 is synthesized almost exclusively in hypothalamus, anterior pituitary and developing ear. Receptor alpha-1 is the first isoform expressed in the conceptus, and there is a profound increase in expression of beta receptors in brain shortly after birth. Interestingly, the beta receptor preferentially activates expression from several genes known to be important in brain development (e.g. myelin basic protein), and upregulation of this particular receptor may thus be critical to the well known effects of thyroid hormones on development of the fetal and neonatal brain.
The presence of multiple forms of the thyroid hormone receptor, with tissue and stage-dependent differences in their expression, suggests an extraordinary level of complexity in the physiologic effects of thyroid hormone.
Interaction of Thyroid Hormone Receptors with DNA
Thyroid hormone receptors bind to short, repeated sequences of DNA called thyroid or T3 response elements (TREs), a type of hormone response element. A TRE is composed of two AGGTCA "half sites" separated by four nucleotides. The half sites of a TRE can be arranged as direct repeats, pallindromes or inverted repeats.
The DNA-binding domain of the receptor contains two sets of four cysteine residues, and each set chelates a zinc ion, forming loops known as "zinc fingers". A part of the first zinc finger interacts directly with nucleotides in the major groove of TRE DNA, while residues in the second finger interact with nucleotides in the minor groove of the TRE. Thus, the zinc fingers mediate specificity in binding to TREs.
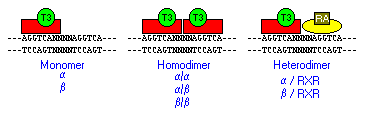
Thyroid hormone receptors can bind to a TRE as monomers, as homodimers or as heterodimers with the retinoid X receptor (RXR), another member of the nuclear receptor superfamily that binds 9-cis retinoic acid. The heterodimer affords the highest affinity binding, and is thought to represent the major functional form of the receptor.
Thyroid hormone receptors bind to TRE DNA regardless of whether they are occupied by T3. However, the biological effects of TRE binding by the unoccupied versus the occupied receptor are dramatically different. In general, binding of thyroid hormone receptor alone to DNA leads to repression of transcription, whereas binding of the thyroid hormone-receptor complex activates transcription.
|
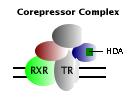
Ligand-free state: The transactivation domain of the T3-free receptor, as a heterodimer with RXR, assumes a conformation that promotes interaction with a group of transcriptional corepressor molecules. A part of this corepressor complex has histone deacetylase activity (HDA), which is associated with formation of a compact, "turned-off" conformation of chromatin. The net effect of recruiting these types of transcription factors is to repress transcription from affected genes. |
 |
|
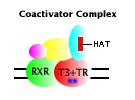
Ligand-bound state: Binding of T3 to its receptor induces a conformational change in the receptor that makes it incompetent to bind the corepressor complex, but competent to bind a group of coactivator proteins. The coactivator complex contains histone transacetylase (HAT) activity, which imposes an open configuration on adjacent chromatin. The coactivator complex associated with the T3-bound receptor functions to activate transcription from linked genes. |
 |
A growing number of specific proteins have been identified as members of the corepressor and coactivator complexes described. It should also be mentioned that there are several exceptions to the scheme described above. As mentioned, the alpha-2 receptor is unable to bind T3 and acts as similarly to a dominant-negative mutant of the receptor, but its carboxy-terminus can be differentially phosphorylated, which affects DNA binding and dimerization. Also, the beta-2 isoform apparently fails to function as a repressor in the absence of T3.
Disorders of Thyroid Hormone Receptors
A number of humans with a syndrome of thyroid hormone resistance have been identified, and found to have mutations in the receptor beta gene which abolish ligand binding. Clinicially, such individuals show a type of hypothyroidism characterized by goiter, elevated serum concentrations of T3 and thyroxine and normal or elevated serum concentrations of TSH. More than half of affected children show attention-deficit disorder, which is intriguing considering the role of thyroid hormones in brain development. In most affected families, this disorder is transmitted as a dominant trait, which suggests that the mutant receptors act in a dominant negative manner.
Mice with targeted deletions in thyroid receptor genes have provided additional understanding of the possible roles of different forms of thyroid hormone receptors. Knockout mice that are unable to produce the alpha-1 receptor showed subnormal body temperature and mild abnormalities in cardiac function. Other mice which lacked expression of both alpha isoforms were severely hypothyroid and died within the first few weeks of life. Mice with disruptions of the entire beta gene exhibited elevated TSH levels and deafness, while mice with mutations that disrupted only beta-2 expression had elevated TSH, but normal hearing. Such experiments are beginning to allow determination of which functions of the different receptor isoforms are redundant and which are not.
Inactivating mutations in thyroid hormone receptors do not produce a syndrome analogous to the lack of thyroid hormones. This is the case even in mice with targeted deletions in both alpha and beta receptor genes. The most likely explanation for the relative mild effects of receptor deficiency is that responsive genes are left in a "neutral" state, rather than being chronically suppressed as happens with hormone deficiency.
References and Reviews
- Brent GA: Mechanisms of disease: The molecular basis of thyroid hormone action. New Eng J Med 331:847-853, 1994.
- Tsai M, O'Malley BW: Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451-486, 1994.
- Zhang J, Lazer MA: The mechanism of action of thyroid hormones. Annu Rev Physiol 62:439-466, 2000.
Send comments to Richard.Bowen@colostate.edu
