VIVO Pathophysiology
Insulin Synthesis and Secretion
Insulin is a small protein, with a molecular weight of about 6000 Daltons. It is composed of two chains held together by disulfide bonds. The figure to the right shows a molecular model of bovine insulin, with the A chain colored blue and the larger B chain green. You can get a better appreciation for the structure of insulin by manipulating such a model yourself.
The amino acid sequence is highly conserved among vertebrates, and insulin from one mammal almost certainly is biologically active in another. Even today, many diabetic patients are treated with insulin extracted from pig pancreas.
Biosynthesis of Insulin
Insulin is synthesized in significant quantities only in beta cells in the pancreas. The insulin mRNA is translated as a single chain precursor called preproinsulin, and removal of its signal peptide during insertion into the endoplasmic reticulum generates proinsulin.
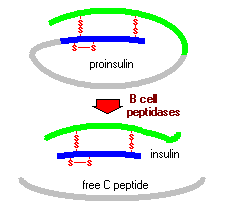 Proinsulin consists of three domains: an amino-terminal B chain, a carboxy-terminal A chain and a connecting peptide in the middle known as the C peptide. Within the endoplasmic reticulum, proinsulin is exposed to several specific endopeptidases which excise the C peptide, thereby generating the mature form of insulin. Insulin and free C peptide are packaged in the Golgi into secretory granules which accumulate in the cytoplasm.
Proinsulin consists of three domains: an amino-terminal B chain, a carboxy-terminal A chain and a connecting peptide in the middle known as the C peptide. Within the endoplasmic reticulum, proinsulin is exposed to several specific endopeptidases which excise the C peptide, thereby generating the mature form of insulin. Insulin and free C peptide are packaged in the Golgi into secretory granules which accumulate in the cytoplasm.
When the beta cell is appropriately stimulated, insulin is secreted from the cell by exocytosis and diffuses into islet capillary blood. C peptide is also secreted into blood, but has no known biological activity.
Control of Insulin Secretion
Insulin is secreted in primarily in response to elevated blood concentrations of glucose. This makes sense because insulin is "in charge" of facilitating glucose entry into cells. Some neural stimuli (e.g. sight and taste of food) and increased blood concentrations of other fuel molecules, including amino acids and fatty acids, also promote insulin secretion.
Our understanding of the mechanisms behind insulin secretion remain somewhat fragmentary. Nonetheless, certain features of this process have been clearly and repeatedly demonstrated, yielding the following model:
- Glucose is transported into the beta cell by facilitated diffusion through a glucose transporter; elevated concentrations of glucose in extracellular fluid lead to elevated concentrations of glucose within the beta cell.
- Elevated concentrations of glucose within the beta cell ultimately leads to membrane depolarization and an influx of extracellular calcium. The resulting increase in intracellular calcium is thought to be one of the primary triggers for exocytosis of insulin-containing secretory granules. The mechanisms by which elevated glucose levels within the beta cell cause depolarization is not clearly established, but seems to result from metabolism of glucose and other fuel molecules within the cell, perhaps sensed as an alteration of ATP:ADP ratio and transduced into alterations in membrane conductance.
- Increased levels of glucose within beta cells also appears to activate calcium-independent pathways that participate in insulin secretion.
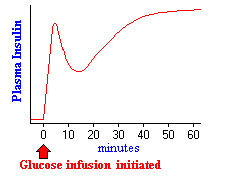 Stimulation of insulin release is readily observed in whole animals or people. The normal fasting blood glucose concentration in humans and most mammals is 80 to 90 mg per 100 ml, associated with very low levels of insulin secretion.
Stimulation of insulin release is readily observed in whole animals or people. The normal fasting blood glucose concentration in humans and most mammals is 80 to 90 mg per 100 ml, associated with very low levels of insulin secretion.
The figure to the right depicts the effects on insulin secretion when enough glucose is infused to maintain blood levels two to three times the fasting level for an hour. Almost immediately after the infusion begins, plasma insulin levels increase dramatically. This initial increase is due to secretion of preformed insulin, which is soon significantly depleted. The secondary rise in insulin reflects the considerable amount of newly synthesized insulin that is released immediately. Clearly, elevated glucose not only simulates insulin secretion, but also transcription of the insulin gene and translation of its mRNA.
Advanced and Supplemental Topics
Send comments to Richard.Bowen@colostate.edu
A Hindi translation of this page was created by Claire Miller from HND Assignment Help and is available at Hindi translation
A Swahili translation of this page was created by Thomas Smith from Assignment Writing Help and is available at Swahili translation

