VIVO Pathophysiology
Other Topics
The Structure of Proteins
Proteins are polymers of amino acids covalently linked through peptide bonds into a chain. Within and outside of cells, proteins serve a myriad of functions, including structural roles (cytoskeleton), as catalysts (enzymes), transporter to ferry ions and molecules across membranes, and hormones to name just a few.
Amino Acids

Proteins are polymers of amino acids joined together by peptide bonds. There are 20 different amino acids that make up essentially all proteins on earth. Each of these amino acids has a fundamental design composed of a central carbon (also called the alpha carbon) bonded to:
- a hydrogen
- a carboxyl group
- an amino group
- a unique side chain or R-group
Thus, the characteristic that distinguishes one amino acid from another is its unique side chain, and it is the side chain that dictates an amino acids chemical properties. Examples of three amino acids are shown below, and structures of all 20 are available. Note that the amino acids are shown with the amino and carboxyl groups ionized, as they are at physiologic pH.
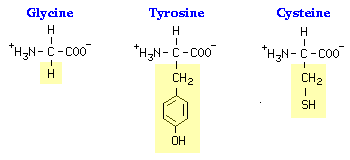
Except for glycine, which has a hydrogen as its R-group, there is asymmetry about the alpha carbon in all amino acids. Because of this, all amino acids except glycine can exist in either of two mirror-image forms. The two forms - called stereoisomers - are referred to as D and L amino acids. With rare exceptions, all of the amino acids in proteins are L amino acids.
The unique side chains confer unique chemical properties on amino acids, and dictate how each amino acid interacts with the others in a protein. Amino acids can thus be classified as being hydrophobic versus hydrophilic, and uncharged versus positively-charged versus negatively-charged. Ultimately, the three dimensional conformation of a protein - and its activity - is determined by complex interactions among side chains.
Peptides and Proteins
Amino acids are covalently bonded together in chains by peptide bonds. If the chain length is short (say less than 30 amino acids) it is called a peptide; longer chains are called polypeptides or proteins. Peptide bonds are formed between the carboxyl group of one amino acid and the amino group of the next amino acid. Peptide bond formation occurs in a condensation reaction involving loss of a molecule of water.
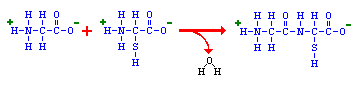
The head-to-tail arrangment of amino acids in a protein means that there is a amino group on one end (called the amino-terminus or N-terminus) and a carboxyl group on the other end (carboxyl-terminus or C-terminus). The carboxy-terminal amino acid corresponds to the last one added to the chain during translation of the messenger RNA.
Levels of Protein Structure
Structural features of proteins are usually described at four levels of complexity:
- Primary structure: the linear arrangment of amino acids in a protein and the location of covalent linkages such as disulfide bonds between amino acids.
- Secondary structure: areas of folding or coiling within a protein; examples include alpha helices and pleated sheets, which are stabilized by hydrogen bonding.
- Tertiary structure: the final three-dimensional structure of a protein, which results from a large number of non-covalent interactions between amino acids.
- Quaternary structure: non-covalent interactions that bind multiple polypeptides into a single, larger protein. Hemoglobin has quaternary structure due to association of two alpha globin and two beta globin polyproteins.
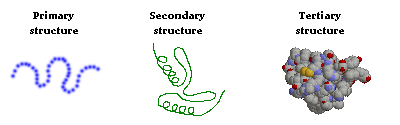
The primary structure of a protein can readily be deduced from the nucleotide sequence of the corresponding messenger RNA. Based on primary structure, many features of secondary structure can be predicted with the aid of computer programs. However, predicting protein tertiary structure remains a very tough problem, although some progress has been made in this important area.
Send comments to Richard.Bowen@colostate.edu