VIVO Pathophysiology
Other Topics
Osmosis: Examples
Practice makes perfect, and shown below are some practice problems on osmosis, osmotic pressure and net flow of water across a selectively-permiable membrane. The membrane allows free passage of water molecules, but does not allow movement of solute particles (molecules and ions) - this is a simple model of a lipid bilayer.
Look at each problem, predict the result, then click the image to see the correct answer and a short explanation of what's going on. One of the goals here is to solidify the idea of describing solutions in terms of molarity versus mass concentration (grams/liter). In the images below MW is an abbreviation for molecular weight (i.e. grams per mole).
Note: If nothing happens when you click on the images below, it means that your browser does not have Javascript enabled, and you will not be able to see the answers.
Example 1: Glucose is a monosaccharide and sucrose (table sugar) is a disaccharide.
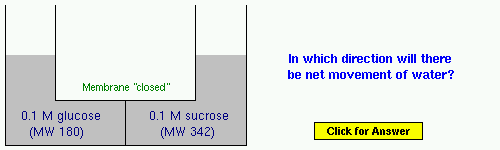
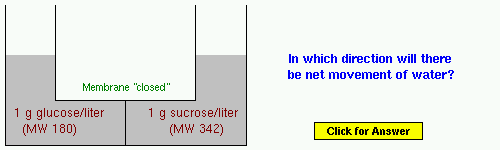
Example 2: Same solutes as in Example 1, but their concentrations are presented differently.
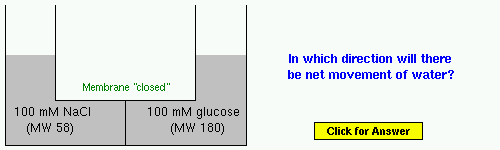
Example 3: NaCl or sodium chloride is, of course, table salt. Before doing the problem, think about what happens to salt when it is dissolved in water.
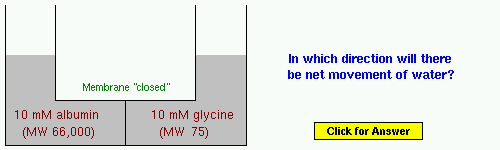
Example 4: Albumin is the most abundant protein in blood. Glycine is an amino acid - assume that it is not a salt.
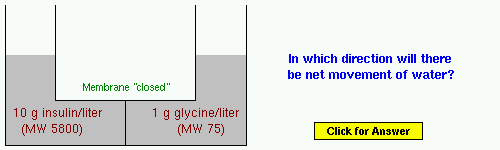
Example 5: Insulin is a small protein hormone that is critical for maintaining normal blood glucose concentrations.
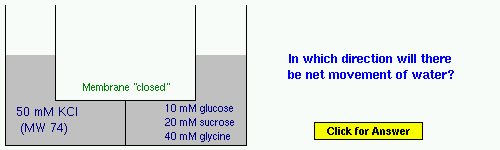
Example 6: KCl or potassium chloride is an inorganic salt. For the first time, we see a mixture of solutes in the right compartment.
Advanced and Supplemental Topics
Send comments to Richard.Bowen@colostate.edu