VIVO Pathophysiology
Other Topics
Adenylyl Cyclase
Adenylyl cyclase is the enzyme that synthesizes cyclic adenosine monophosphate or cyclic AMP from adenosine triphosphate (ATP). Cyclic AMP functions as a second messenger to relay extracellular signals to intracellular effectors, particularly protein kinase A. Regulation of intracellular concentrations of cyclic AMP is largely a result in controlling adenylyl cyclase.
Structure and Function
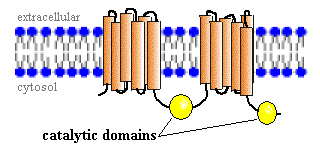
Adenylyl cyclases are integral membrane proteins that consist of two bundles of six transmembrane segments. Two catalytic domains extend as loops into the cytoplasm, as depicted in the figure to the right.
A soluble (non-membrane bound) form of adenylyl cyclase has recently been characterized in mammalian sperm. This form of the enzyme appears to be activated by bicarbonate ion.
When adenylyl cyclase is activated, it catalyses the conversion of ATP to cyclic AMP, which leads to an increase in intracellular levels of cyclic AMP.
Regulation of Activity
There are at least nine isoforms of adenylyl cyclase, discovered by cloning of full-length cDNAs. These enzymes differ considerably in regulatory properties and are differentially expressed among tissues, adding support to observations that support a very complex model of interactions that regulate cyclic AMP production.
Early studies indicated that cyclase activity was regulated primarily by interactions with alpha subunits of heterotrimeric G proteins, which are activated through G protein-coupled receptors. Binding of a stimulatory G alpha (Gs) enhanced activity while binding of an inhibitory G alpha (Gi) inhibited cyclase activity. This is certainly the case in some situations. For example, the beta-adrenergic receptor is coupled to adenylyl cyclase via Gs and binding of epinephrine to this receptor leads to increased cyclic AMP synthesis. Also, when epinephrine binds to alpha-2 adrenergic receptors, adenylyl cyclase activity is inhibited, because that receptor is coupled to via Gi, an inhibitory G protein.
More recently, it has become clear that cyclase activity is regulated by multiple effectors, which include not the alpha subunits of Gs and Gi proteins, but also the beta-gamma subunits of G proteins and protein kinase C.
Of potentially great significance, five of the adenylyl cyclases known are regulated by calcium. Three of these are stimulated by calcium and two are inhibited. Also, ultrastructural labelling has demonstrated a close spatial association of adenylyl cyclases with sites of calcium entry in cells. It thus appears that there is tight integration between cAMP and calcium, the cell's two major internal signallers.
All known adenylyl cyclases are stimulated by exposure of cells to the diterpene forskolin. This drug is widely used in studies aimed at dissecting intracellular signalling pathways.
References and Reviews
- Chen Y, Cann MJ, Litvin TN, etc: Soluble adenylyl cyclase as an evolutionarily-conserved bicarbonate sensor. Science 289:625-628, 2000.
- Cooper DMF, Mons N, Karpen JW: Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature 374:421-424, 1995.
- Houslay MD, Milligan G: Tailoring cAMP-signalling responses through isoform multiplicity. Trends Biochem Sci 22:217, 1997.
- Mons N, Decorte L, Jaffard R, Cooper DM: Ca2+-sensitive adenylyl cyclases, key integrators of cellular signalling. Life Sciences 62:1647, 1998.
- Smit MJ, Iyengar R: Mammalian adenylyl cyclases. Advances in Second Messenger and Phosphoprotein Res 32:1, 1998.
Send comments to Richard.Bowen@colostate.edu